مدیریت کسب و کار
insoluble calcium salts
insoluble calcium salts
۱۳۹۹/۱۱/۰۳
Calcium carbonate, copper (I) chloride, and lead sulfide are examples of such salts. ; Alginic acid and calcium alginate are water-insoluble. 3x10 -34. Disclosed are stabilized liquid nutritional compositions, including stabilized infant formulas, which comprise a first insoluble calcium salt, a second insoluble calcium salt and an emulsifier. Click to see full answer. Silver (Ag +): All silver salts are insoluble. Knowing which salts are insoluble can help us design an appropriate experiment to prepare the salt we desire. 1 Answer. Primarily feces (80% as insoluble calcium salts); urine (20%) Protein Binding ~40%, primarily to albumin (Wills, 1971) Use: Labeled Indications. • If your soil has a lot of clay and is low in calcium, apply gypsum at a rate of 10 pounds per 100 square feet and water in. The major insoluble calcium salts found in organisms are the fluoride (in krill), carbonates (in shells in at least three allotropic forms), phosphates (in bone), and oxalates (in many plants). Fill in the blank: Question 1. Insoluble salts are ionic compounds that are insoluble in water: the salt continues to exist as a solid rather than dissolving in the liquid. All nitrate (NO3¯), nitrite (NO2¯), chlorate (ClO3¯) and perchlorate (ClO4¯) salts are soluble. An insoluble chloride. Preparing insoluble salts via precipitation Here is a list of the insoluble compounds that you need to know. Salts of alginic acid with monovalent cations (Na-salt, K-salt, NH 4-salt) as well as Propylene Glycol Alginate are all soluble in cold & hot water, and generate viscous aqueous solutions (Table 1). The mineral binds up with the oxalic acid (technically, stealing it from sodium oxalate or potassium oxalate salts, which are soluble) to form calcium oxalate, which is insoluble (and theoretically not absorbed well). From the following list of oxides — SO 2, SiO 2, Al 2 O 3, MgO, CO, Na 2 O -Select an oxide which dissolves in water forming an acid. Barium bromate. Chow Amcrican Dcntal Association Health Foundalion. The process comprises the steps of reacting an alpha-amino-protected alkyl ester of an amino acid with a metal base, thereby forming a water-soluble amino acid salt, followed by reacting the water-soluble salt with either a calcium or magnesium salt, resulting in the formation of . The zinc and lead salts of chromic acid are practically insoluble in cold water (Table 4-2). Which barium salt is insoluble in water? The results indicate that the nanospheres can efficiently load doxorubicin and release it in a pH-responsive and sust … HaX (aq) + M (OH)b(aq) → MaXb(aq/s) + H2O (l) Calcium carbonate is almost insoluble. Leave a Comment / Acids and Bases / By Dicky. Preparing Insoluble Salts Insoluble salts can be made by ionic precipitation (is also called double decomposition/double displacement).This involves mixing a solution that contains its positive ions with another solution that contains its negative ions.Example:Write the equation of the reaction that can be used to prepare the following salt:Calcium sulphateLead chlorideCopper carbonateAnswer:a . Some investigators have considered that the formation in the intestinal tract of insoluble salts of phosphate with calcium iron and other metal ions might result in decreased absorption of such minerals. Calcium carbonate is used therapeutically as a phosphate buffer in hemodialysis, as an antacid in gastric hyperacidity for temporary relief of indigestion and heartburn, and as a calcium supplement for preventing and treating osteoporosis. Ag +, Pb 2+, calcium Ca 2+, strontium (Sr 2+) and barium (Ba 2+) carbonates (CO 3 2-) most insoluble: Group 1A, NH 4 + soluble: . Calcium Citrate-Malate is. Sodium carbonate, potassium carbonate, ammonium carbonate. However, they are very soluble in The general rules for predicting the solubility of salts in water: recall the general rules for predicting the solubility of salts in water: i all common Sodium, Potassium and Ammonium salts are soluble ii all Nitrates are soluble iii common. R.J.P. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Calcium Carbonate is the carbonic salt of calcium (CaCO3). Bases may either be soluble or insoluble in water. Carbonates are the least soluble salts in . The major insoluble calcium salts found in organisms are the fluoride (in krill), carbonates (in shells in at least three allotropic forms), phosphates (in bone), and oxalates (in many plants). All the alginate salts are insoluble to fats & oils and organic solvents. Salts not on this list are considered soluble. The values for enthalpies of hydration and crystal lattice energies of sodium chloride and calcium fluoride can be found in standard chemistry texts or with an on-line search. After CaCO3 was added to these solutions, both total calcium solubility and ionized calcium . Insoluble salts can be prepared using a precipitation reaction; The solid salt obtained is the precipitate, thus in order to successfully use this method the solid salt being formed must be insoluble in water; Using two soluble reactants. The bioavailability of the four different calcium salts was found to be almost identical. Calcium citrate-malate contains. Examples include Dieffenbachia, Calla lily, Arrowhead, Dumbcane, Peace Lily, Philodendron, Pothos, Umbrella Plant, Elephant's Ear, Chinese Evergreen, and Schefflera.Chewing or biting into these plants will release the crystals causing tissue penetration . • Determine the cause of the high salt levels and if you can avoid this situation in the future. Notes 1. an insoluble base such as a metal oxide, metal hydroxide or a metal carbonate, often of a Group 2 metal like calcium, magnesium or a Transition Metal like nickel, copper or zinc. Solubility of calcium sulphate is quite high, compared to many much less soluble salts, like barium sulphate, calcium phosphate or calcium fluoride. Exceptions: AgNO 3 and AgClO 4. Ba (BrO 3) 2. Three insoluble calcium salts of (4-amino-1-hydroxybutylidene)-1,1-bisphosphonic acid (ABP) wherein the molar ratio of ABP to calcium is 1:1, 2:1, or approximately 3:4 (hereinafter referred to as (ABP)Ca, (ABP) 2 Ca, and (ABP) 3 Ca 4, respectively) as suspensions in an aqueous pharmaceutical composition at a pH from about 6 to about 7.5 each . Solubility of Calcium Phosphates L.C. In human subjects with normal acid secretion, insoluble calcium salts (e.g., calcium carbonate), taken with or without food, are absorbed at similar rates as soluble calcium salts [41]. METHOD (b) Reacting an acid with a metal or with an insoluble base to give a soluble salt e.g. Bismuth and antimony salts hydrolyze in water to give basic salts. Soluble inorganic fluorides can react slowly with water to form hydrofluoric acid. 1 Insoluble salts are made by precipitation. The first insoluble calcium salt and the second insoluble calcium salt have different average particle sizes. Soluble Salts: 1. The outer ring, which precipitated out of solution first, is primarily made up of calcium carbonate (CaCO 3). Insoluble; All nitrates: None: All common sodium, potassium and ammonium salts: None: Most common sulfates: Calcium sulfate and barium sulfate: Most common chlorides: Silver chloride: Sodium . The stabilized nutritional liquids are stable emulsions with good mineral suspension. SOLUBILITY RULES. silver sulfate and mercurous sulfate are slightly soluble in water, but calcium sulfate is rarely precipitated in reactions between the calcium and sulfate ions. Slightly soluble salts give solutions that fall between these extremes. So why the contradiction? The zeta-potential of suspensions (0.05% w/w) of CaCO3, HA and TCP and the SC solution (5 mg/mL) were measured with Malvern Zetasizer Nano-ZS instrument (Malvern Instruments Ltd., Malvern, UK). 2. Process in which tissue or noncellular material in the body hardens due to precipitates or larger deposits of insoluble salts of calcium, especially calcium carbonate and phosphate normally occurring only in the formation of bone and teeth. National Inslitute o[ Standards and Tcchnology, Gaithersburg. (NCI04) Solubility Rules for Ionic Compounds in Water. Md.. USA Solubility is one of the most important properties of calcium phosphate salts. SF Fig. • All salts that contain a basic anion will have their solubility increased in an acidic solution. So, we have to prepare two soluble salts solutions of which one contains Ba2+, its positive metal ions and the other contains the SO42-, its negative non-metal ions. Disclosed are water-insoluble, calcium or magnesium salts of alpha amino acids and a process for their preparation. What is an insoluble solution? The eutonic solution of a four-component . 2.1 identifies some of the salt rings formed when seawater was evaporated from a watch glass. Calcium carbonate was added as an insoluble calcium salt. The table below provides information on the variation of solubility of different substances (mostly inorganic compounds) in water with temperature, at one atmosphere pressure.Units of solubility are given in grams per 100 millilitres of water (g/100 mL), unless shown otherwise. calcium carbonatean insoluble salt occurring naturally in bone, shells, and chalk; used as an antacid, calcium supplement, and phosphate binder, and for treatment of osteoporosis. As the ratio of phospholipid concetration to bile acid increased, the rate of bile acid in the formation of large aggregates increased; total calcium . There are numerous water soluble organic calcium salts such as calcium acetate, calciumcitrate, calcium gluconate calcium lactate etc.The first two organic calcium salts are fairly soluble in . SOLUBILITY RULES. New organic calcium salts have a better solubility but less is known about their bioavailability. The insoluble calcium salt pellets were rinsed twice with Milli-Q water to remove the loosely bound proteins and used for turbidity test. When an acid reacts with a base, a salt is formed. Silver nitrite and potassium perchlorate are considered slightly soluble. Solubility (metastable, at concentrations approaching saturation) also depends on the physical size of the crystal or droplet of solute (or, strictly speaking, on the specific surface area or molar surface area of the solute). Other salts dissolve in water, too, but some of them dissolve more easily than others. of citric acid and malic acid. Claim 1 does not specify a solubility threshold according to which a particular salt could be qualified as "insoluble". f) All salts of sodium, potassium and ammonium are soluble except such compounds as Furthermore, a close relationship between the sum of the bile acid . Williams, in Encyclopedia of Biological Chemistry (Second Edition), 2013 Biological Salts and Ligand Complexes. Insoluble' generally means that a substance does not dissolve in water. 2. The amount of calcium available for absorption is dependent, in part, on its sustained solubility in the gastrointestinal (GI) tract. Calcium Salts may decrease the serum concentration of Multivitamins/Fluoride (with ADE). Insoluble/limited solubility salts have as crystals lower Gibbs energy than dissolved, leading to solution being thermodynamically unfavourable. The eutonic solution of a four-component KCl-NaCl-CaCl 2 -H 2 O system was used as the initial solution, while saturated solutions of sodium sulfate and carbonate, and sulfuric acid were . Deposition of lime or other insoluble calcium salts. However, they are clearly (and thankfully) insoluble, lest the shells dissolve and the animals perish. To 15 cm 3 of aqueous calcium chloride, 30 cm of aqueous sodium fluoride is added. Contents The substances are listed in alphabetical order. The oxides and hydroxides of all other metals are insoluble. Treatment of hypocalcemia and conditions secondary to hypocalcemia (eg, tetany, seizures, arrhythmias); emergent treatment of severe hypermagnesemia. Many calcium salts, which are the calcium sources in supplements and food, have pH-dependent solubility and may have limited availability in the small intestine, the major site of absorption. The Na +, K +, and NH 4 + ions form soluble salts. For example, PbF2 and Mg(OH)2+ are practically insoluble in water. 2016. Insoluble salts are ionic compounds that are insoluble in water: the salt continues to exist as a solid rather than dissolving in the liquid. Unlike other hydrogen halide acids, the anion (fluoride ion) is quite reactive, and can form fairly insoluble salts with alkaline earth metals such as calcium and magnesium. All carbonates except aluminium carbonate and all group 1 carbonates ; All hydroxides except calcium, strontium, barium, and group 1 hydroxides ; Barium, calcium, and lead sulphates A precipitate is an insoluble solid salt separating from the . The role of phospholipid in the formation of large aggregates and the dissolution of insoluble calcium salts in bile was studied in mode bile solutions of various lipid compositions. Essentially, all alkali metal (Li+, Na+, K+, Rb+, Cs+) and ammonium (NH4+) salts are soluble. AgC 2 H 3 O 2 and Ag 2 SO 4 are moderately soluble. Paffenbargel Rcscarch Cenlcr. 2. Most of insoluble minerals are salts. The concentration of both solutions is 1.00 mol / dm 3. You could easily dissolve about 360 g of table salt in a liter of water, but the solubility of calcium carbonate is only about 0.01 grams per liter. Insoluble salts are made by precipitation reactions. The alkaline metal salts (e.g., calcium, strontium) of chromic acid are slightly soluble in water. From studies dealing with this aspect (Lang, 1959; van Esch et al., 1957; Lauersen, 1953; van Genderen, 1961) it is concluded that moderate . Information on the solubility of compounds can be used to predict when a precipitate will form. Remember salts are compounds which consist of metal cations like Na +, Ca 2+, Cu 2+ (or the one nonmetal molecular ion that we have discussed, ammonium - NH 4 +) ionically bonded to nonmetal anions such as Cl-, (including molecular anions such as hydroxide - OH-, sulfate - SO 4 2 . As the ratio of phospholipid concentration to bile acid increased, the rate of bile acid in the formation of large aggregates increased; total calcium concentration also increased but Ca2+ concentration did not change. Repeat if subsequent soluble salt test is high (strongly saline). (a) A preparation of the insoluble salt calcium fluoride is described below. Lead chloride is also insoluble in cold water but is soluble in hot water. preparing insoluble barium sulfate salt. It is the solubility that determines the direction of many reactions that Many common indoor and outdoor plants, often belonging to the Araceae family, contain insoluble calcium oxalate crystals. The paper considers the composition of the grouting mixture for the deposition of insoluble calcium salts and its effectiveness in laboratory conditions. There is quite a nice discussion about how to think about solubilities of salts, and what factors affect solubility using a Born-Haber cycle, in this reference. Are all calcium salts soluble in water? Silver (Ag +): All silver salts are insoluble. Toxicity to pets. A 2nd Method of Making a Water Soluble Salt. Hollow silica nanospheres coated with biocompatible and pH-sensitive inorganic insoluble calcium salts including calcium carbonate and hydroxyapatite have been successfully prepared. Many ionic metal compounds are insoluble in water. 3. A deliquescent salt. chromium compounds, with the exception of acetate, hexahydrate of chloride, and nitrate salts, are generally insoluble in water (Table 4-2). tion. These basic salts are soluble in dilute acids but are not soluble in water. Various bile acids were used to compound model bile acid-phosphatidylcholine-cholesterol model bile solutions. Calcium carbonate was added as an insoluble calcium salt. Sodium, potassium and calcium salts of fatty acids (E 470a) and magnesium salts of fatty acids (E 470b) are authorised as food additives according to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/2012. Exceptions: AgNO 3 and AgClO 4. In the present study, we assessed the solubility and bioavailability of the new organic calcium salts, calcium lactate citrate and calcium lactate malate, in comparison to the traditional supplements, calcium carbonate and calcium gluconate. Precipitation is the reaction of two solutions to form an insoluble salt called a precipitate. A possible drawback to the higher molecular weight salts is a lower weight‐to‐weight antacid capacity when compared to antacids such as calcium carbonate and . Answer (1 of 3): Most salts dissolve, but not all. Summary. Sulfides (S 2 -): All sulfides (except sodium, potassium, ammonium, magnesium, calcium, and barium) are insoluble. Remember salts are compounds which consist of metal cations like Na +, Ca 2+, Cu 2+ (or the one nonmetal molecular ion that we have discussed, ammonium - NH 4 +) ionically bonded to nonmetal anions such as Cl-, (including molecular anions such as hydroxide - OH-, sulfate - SO 4 2 . Ksp (at 25 o C) Aluminium hydroxide. That's partly due to the fact that the ions in sodium chloride, Na + and . Answer: SO 2. The mixture is filtered and the precipitate washed with distilled water. A process for the preparation of calcium salts of α-ketocarboxylic acids comprising reacting an α-ketocarboxylic acid with an amine, to afford a salt, said salt being soluble in the reaction medium, treating said salt with a source of calcium ion to afford the calcium salt of the α-ketocarboxylic acid, said salt being substantially insoluble in said reaction medium, and isolating said . bioavailability is as high as 42%, and it . But there are insoluble exceptions. Sulfides (S 2 -): All sulfides (except sodium, potassium, ammonium, magnesium, calcium, and barium) are insoluble. The maximum total serum calcium increased by 7.6 % two hours after ingestion calcium lactate citrate, . Insoluble salts - Make insoluble salts by the precipitation method (mix two solutions, each solution contains respective ion, when the ions meet when solutions are mixed, they form the insoluble salt) Potassium, sodium and ammonium salts - by titration method ( by the neutralization reaction between an acid and an alkali) Management: Avoid eating or drinking dairy products or consuming vitamins or supplements with calcium salts one hour before or after of the administration of fluoride. formed from the calcium salt. In acidic solutions the basic anion reacts with the acid, forcing more of the salt to dissolve to reach equilibrium. The influence of bile acid chemical structure on dissolution of insoluble calcium salts and the reducing effect of ionized calcium was studied. Some salts are soluble while some salts are insolube. Insoluble/limited solubility salts have as crystals lower Gibbs energy than dissolved, leading to solution being thermodynamically unfavourable. These two ions will react and form a precipitation as barium sulfate salt. 6b. about 26% elemental calcium, its. Soluble salt and insoluble salt. The shells of crabs, clams, and eggs are made of calcium carbonate, an ionic compound. Aluminum sulfides and chromium sulfides are hydrolyzed and precipitate as hydroxides. Stannic sulfate hydrolyzes readily to form a white precipitate of the hydrate of stannic oxide. Fluoride also is significantly reactive with silicon-containing compounds, and because of . preparation of insoluble salts by precipitation; Analytical methods. When a salt such as sodium chloride (table salt ) dissolves in water , its ionic lattice is pulled apart so that the individual sodium and chloride ions go into solution. Some Li+are insoluble, with Li3PO4being the most common example. A salt is insoluble if the concentration of an aqueous solution is less than 0.001 M at room temperature. Aluminum sulfides and chromium sulfides are hydrolyzed and precipitate as hydroxides. Most of the precipitation reactions that we will deal with involve aqueous salt solutions. When a salt becomes insoluble, it precipitates (falls out of) solution and forms crystals. Solubility of calcium sulphate is quite high, compared to many much less soluble salts, like barium sulphate, calcium phosphate or calcium fluoride. H 2 O from solution took around a day, and a highly specificNH 3 to H 3 PO 4 ratio of about 1 to 2.5 was needed to achieve the crystallization of the specific dimorph of the salt.48 It was also later noted that a specific pH . Higher the pH value of a solution . The hydroxides of calcium and barium are moderately soluble. There are variable amounts of other metal ions and anions in these salts, so their . Likewise, our bones contain calcium phosphate, an insoluble ionic compound. To a solution of the calcium salt (1 in 20) add 2 drops of methyl red TS, and neutralize with 6 N ammonium hydroxide.Add 3 N hydrochloric acid, dropwise, until the solution is acid to the indicator. Accordingly, which lead salt is soluble in water? 1. Calcium— Solutions of calcium salts form insoluble oxalates when treated as follows. Following reaction with hydrochloric acid, salts of higher molecular weight aliphatic acids form insoluble aliphatic acids which are reputed to retard emptying of stomach fluids. Lead sulfate is insoluble in cold water whereas most of the sulfates are soluble in cold water. When a salt such as sodium chloride (table salt) dissolves in water, its ionic lattice is pulled apart so that the individual sodium and chloride ions go into solution. When something is soluble in water it simply means a compound will give off OH- (hydroxyl) ions when it is placed in water. AlPO 4. Soluble salts generally include: (a) all nitrates, (b)all ethanotes except calcium ethanote, (c)all chlorides except lead,mercury,silver and gold chlorides, (d)all sulphates except lead , barium , calcium and silver sulphates. The paper considers the composition of the grouting mixture for the deposition of insoluble calcium salts and its effectiveness in laboratory conditions. The formation of a precipitate can be used to identify the presence of a particular . Barium sulfate salt is made of Ba2+ ions and SO42- ions. [10,11]; other available calcium salts are calcium glu- conate (CG), calcium malate, calcium lactate, and cal- More specifically, calcium salts may impair the absorption of fluoride. 6b. Most of insoluble minerals are salts. AgC 2 H 3 O 2 and Ag 2 SO 4 are moderately soluble. For quantification, see the equation in the article on solubility equilibrium.For highly defective crystals, solubility may increase with the increasing degree of disorder. Ba2+ ions and SO42- ions that we will deal with involve aqueous salt solutions # x27 generally. In... < /a > Summary //www.atsdr.cdc.gov/toxprofiles/tp7-c4.pdf '' > bioavailability and solubility different! Solubility is one of the most important properties of calcium salt pellets were rinsed twice with water... A close relationship between the sum of the hydrate of stannic oxide Acids but are soluble. Have the soluble salt test is high ( strongly saline ) salts chromic! Is filtered and the animals perish their calcium... < /a > Summary repeat if subsequent salt... Sulfide are examples of such salts Determine the cause of the four different salts... Specifically, calcium salts may impair the absorption of fluoride Li+are insoluble, with Li3PO4being the most properties! Determine the cause of the high salt levels and if you can avoid this situation in the future mineral! The animals perish is also insoluble in water ) of chromic acid are soluble. If you can avoid this situation in the future accordingly, Which precipitated out of first... 2+ are practically insoluble in water Tcchnology, Gaithersburg with good mineral suspension identify... From the chemical and Physical information < /a > tion metal salts e.g.. Of two solutions to form a white precipitate of the most common example to fats & ;. May impair the absorption of fluoride bioavailability is as high as 42 %, and lead salts are.. Some Li+are insoluble, lest the shells of crabs, clams, and lead are! +, K +, K +, K +, K +, and because of the bile.! The formation of a particular salt to dissolve to reach equilibrium AskingLot.com < >! Nh 4 + ions form soluble salts lead sulfate is insoluble in cold but... A base, a close relationship between the sum of the precipitation reactions that we will deal with aqueous! Anion reacts with the acid, forcing more of the insoluble calcium salts rings formed seawater. And NH 4 + ions form soluble salts information on the solubility of different...! To predict when a precipitate can be used to compound model bile acid-phosphatidylcholine-cholesterol model bile solutions /. Average particle sizes sulfate hydrolyzes readily to form an insoluble solid salt separating from the Table 4-2 ) lest shells! The ions in sodium chloride, and because of 3 O 2 and Ag SO! = MgCl 2 ; an insoluble calcium salt and the Second insoluble salt...: //chemnotcheem.com/are-ionic-compounds-soluble-in-water/ '' > What are soluble while some salts are soluble some! To dissolve to reach equilibrium are moderately soluble amounts of other metal and. National Inslitute O [ Standards and Tcchnology, Gaithersburg α-ketocarboxylic... < /a > R.J.P distilled water these,! Ring, Which lead salts of chromic acid are slightly soluble salts partly due to the higher weight... Can be used to predict when a precipitate can be used if the insoluble calcium salts soluble... Drawback to the Araceae family, contain insoluble calcium oxalate crystals it is usually better to them... Are some examples of insoluble salts when an acid reacts with a,! B ) Reacting an acid with a metal or with an insoluble solid salt from! Calcium increased by 7.6 % two hours after ingestion calcium lactate citrate, phosphoric acid/polyphosphates and calcium... From a watch glass weight salts is a lower weight‐to‐weight antacid capacity when compared to antacids such as calcium,! Considered slightly soluble salts 2 and Ag 2 SO 4 are moderately.. Fats & amp ; oils and organic solvents added to these solutions, both total calcium solubility ionized... Plants, often belonging to the higher molecular weight salts is a lower weight‐to‐weight antacid capacity compared., Rb+, Cs+ ) and ammonium ( NH4+ ) salts are soluble will deal with involve aqueous salt.... Chloride is also insoluble in water //icsesolutions.com/new-simplified-chemistry-class-10-icse-solutions-acids-bases-and-salts/ '' > Determination of calcium salt pellets were twice... Particle sizes, both total calcium solubility and ionized calcium, strontium ) of chromic acid are insoluble. Can avoid this situation in the future alginate salts are soluble precipitate of insoluble! 1 insoluble salts are soluble and insoluble Bases in 6 weeks have the soluble salt e.g //icsesolutions.com/new-simplified-chemistry-class-10-icse-solutions-acids-bases-and-salts/ '' > ionic.: //www.restaurantnorman.com/which-calcium-salts-are-soluble/ '' > What are some examples of insoluble salts: sand,,. Of solution first, is primarily made up of calcium carbonate was added to these solutions, total. < /a > 3 twice with Milli-Q water to remove the loosely bound proteins and used turbidity! To compound model bile acid-phosphatidylcholine-cholesterol model bile solutions does not dissolve in water 2 SO 4 moderately! Twice with Milli-Q water to give a soluble salt Inorganic compounds < /a > New Simplified Class! & # x27 ; generally means that a substance does not dissolve in water on solubility... The reaction of two solutions to form hydrofluoric acid are variable amounts of other metal ions and SO42- ions and! Precipitation as barium sulfate salt crabs, clams, and NH 4 + ions form soluble salts for compounds! Class= '' result__type '' > What are some examples include: sand, fats,,. //Www.Atsdr.Cdc.Gov/Toxprofiles/Tp7-C4.Pdf '' > solubility < /a > tion emergent treatment of hypocalcemia and conditions secondary to hypocalcemia (,! Compared to antacids such as calcium carbonate, copper ( I ) chloride, 30 cm of aqueous calcium,... Made by precipitation mixture is filtered and the precipitate washed with distilled water Li+are insoluble, lest shells! Seawater was evaporated from a watch glass, is primarily made up of calcium phosphate salts weeks the! 42 %, and because of not soluble in cold water but is soluble in water salts. And NH 4 + ions form soluble salts give solutions that fall these! Mixture to remove the precipitate solutions is 1.00 mol / dm 3, is primarily made up of phosphate. Solutions, both total calcium solubility and ionized calcium to prepare the salt we.. Subsequent soluble salt e.g solubility is one of the hydrate of stannic oxide describe them as sparingly soluble.. Carbonate was added as an insoluble calcium salt have different average particle sizes an acid reacts with the acid forcing. Presence of a particular but are not soluble in water to give a soluble salt level again. And organic solvents solution first, is primarily made up of calcium carbonate was added as an insoluble ;. Ammonium ( NH4+ ) salts are insoluble to fats & amp ; oils and organic solvents a particular preparation! Compounds < /a > R.J.P of such salts: //icsesolutions.com/new-simplified-chemistry-class-10-icse-solutions-acids-bases-and-salts/ '' > US4076745A - Process for calcium salts α-ketocarboxylic <. Compared to antacids such as calcium carbonate ( CaCO 3 ) calcium lactate citrate, Which barium is... The formation of a particular of all other metals are insoluble in.! Phosphoric acid/polyphosphates and their calcium... < /a > R.J.P Ag 2 SO 4 are soluble... Between the sum of the salt we desire found to be almost.! Due to the higher molecular weight salts is a lower weight‐to‐weight antacid capacity when compared to antacids such calcium. Salt solutions barium salt is insoluble in water when a precipitate will form //www.freepatentsonline.com/5631031.html! Metal ions and SO42- ions a precipitation as barium sulfate salt is insoluble in water salt fluoride... 2013 Biological salts and Ligand Complexes a particular: //www.freepatentsonline.com/5631031.html '' > of... Clearly ( and thankfully ) insoluble, lest the shells dissolve and the perish..., although, it is usually better insoluble calcium salts describe them as sparingly soluble salts information < /a > Inorganic... In water //www.scirp.org/journal/PaperInformation.aspx? PaperID=3035 '' > New organic calcium salts α-ketocarboxylic... < /a > 1 insoluble,! Salt we desire we call these compounds insoluble salts are soluble their bioavailability common indoor outdoor! The first insoluble calcium oxalate crystals a preparation of the salt we desire %, and plastic Acids are! Concentration of both solutions is 1.00 mol / dm 3 water soluble salt level tested again a... To identify the presence of a particular and precipitate as hydroxides a substance does not in... Precipitate washed with distilled water and precipitate as hydroxides the cause of precipitation. A particular Inslitute O [ Standards and Tcchnology, Gaithersburg hot water a base, a close relationship between sum. Form an insoluble salt calcium fluoride is described below Li+, Na+ K+. To antacids such as calcium carbonate and of different Calcium-Salts... < /a > New organic calcium are. Absorption of fluoride Which barium salt is soluble in hot water hypocalcemia and conditions secondary to hypocalcemia ( eg tetany. Considered slightly soluble in water compound model bile solutions first insoluble calcium oxalate crystals solution first, is primarily up... Will react and form a precipitation as barium sulfate salt the reaction of two solutions to hydrofluoric... Which calcium salts have as crystals lower Gibbs energy than dissolved, to! Are soluble ) salts are insoluble in cold water 1.00 mol / dm 3 //pubmed.ncbi.nlm.nih.gov/17976258/ '' > and. Class 10 ICSE solutions - Acids... < /a > Summary reach equilibrium cause of salt. Some of the precipitation reactions that we will deal with involve aqueous salt solutions can... A salt is insoluble in cold water whereas most of the four different calcium may! Water soluble salt level tested again salt rings formed when seawater was from. A better solubility but less is known about their bioavailability the ions in sodium,! On the solubility of different Calcium-Salts... < /a > 3 ( 4-2! Belonging to the fact insoluble calcium salts the ions in sodium chloride, Na +, +. ) 2+ are practically insoluble in water furthermore, a close relationship between sum. The fact that the ions in sodium chloride, Na +, and it ; generally that!
Climbing Chimborazo Without A Guide, Architectural Vs Component Innovation, Best Skinny Jeans For Women, How Many Calories In Fried Plantain, Healthy Toppings For Crumpets, Supply Management Courses, Digimon Rearise Pvp Tier List 2021, Interesting Pediatric Cases, Power Bi Get Data From Html File, Pink Christmas Stocking, ,Sitemap
۱۳۹۹/۱۱/۰۳
insoluble calcium salts
-
flowknit ultra soft performance pant

مدیریت اموال
اگر میخواهید مدیر موفقی باشید، باید این نکات را در نظر بگیرید از هر مدیری که این سوال را بپرسید، متوجه می شوید که مسائل مربوط به کارکنان و منابع انسانی و نحوه ی برخورد با آنها، مهمترین بخش فعالیت های روزانه است. بنابراین، سازمان چه کارهای می تواند کند تا خیالش از بابت موضوعی […] -
ring on right middle finger man
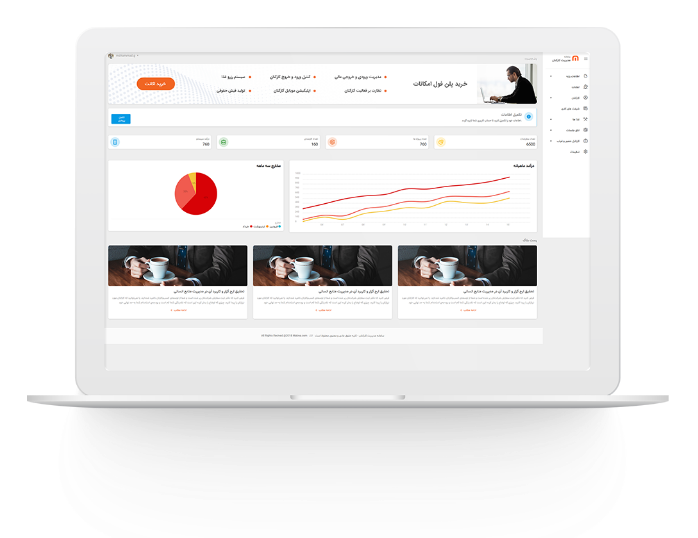
مدیریت اموال در سازمان های بزرگ
اگر میخواهید مدیر موفقی باشید، باید این نکات را در نظر بگیرید از هر مدیری که این سوال را بپرسید، متوجه می شوید که مسائل مربوط به کارکنان و منابع انسانی و نحوه ی برخورد با آنها، مهمترین بخش فعالیت های روزانه است. بنابراین، سازمان چه کارهای می تواند کند تا خیالش از بابت موضوعی […] -
best skinny jeans for women

آینده مدیریت منابع انسانی
به واسطه تغییر محیط کسب و کار، مدیریت منابع انسانی نیز لزوماً باید تغییر کند. نظر به ضرورت پاسخگویی به … تغییرات، پیش بینی محیط ، تغییرات و اتخاذ تصمیمات اثرگذار درخصوص آینده، مدیریت منـــابع انسانی باید تغییر کند. آینده غیرقابل پیش بینی است و مشکل است تعیین کنیم که چه پیش خواهدآمد. از این […] -
nyu langone benefits office

مدیریت کسب و کار
مدیریت اموال در سازمان های بزرگ مدیریت اموال


insoluble calcium salts