مدیریت کسب و کار
electrolysis of calcium fluoride balanced equation
electrolysis of calcium fluoride balanced equation
۱۳۹۹/۱۱/۰۳
At the initial stage of industrialization (1784 ∼ 1870), the voltaic pile (Zn-Cu) was invented in 1799 and gave birth to a series of other important discoveries, such as the electrolysis to separate sodium (1807), potassium (1807), and calcium (1808). It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. Carbon dioxide, CO 2, and silicon dioxide, SiO 2, both occur widely in nature. Limewater traditionally means a weak solution of the alkali calcium hydroxide Ca(OH) 2. Balancing It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. They also have some similar physical properties; for … Calcium phosphate is present in teeth and Calcium carbonate is present in bones. write a balanced chemical equation for the reaction, assuming that the complex is … Rechargeable aqueous Zn-based energy storage devices ... Write the balanced equation for the formation of calcium propionate. … The total number of fluoride ions present in three moles of BaF2 is equal to 6 x(6.02 x 1023). a) 6 b) 19 c) 17 d) 12. Academia.edu is a platform for academics to share research papers. Ex) calcium + oxygen ( calcium oxide (ii) 1 mole H 2 S gas consumes 1 mole Cl 2 gas. When CO 2 is passed through limewater, it reacts with calcium hydroxide to form insoluble particulates (precipitate of calcium carbonate (CaCO 3). Hence, 112 cm 3 H 2 S gas will produce 112 × 2 = 224 cm 3 HCl gas. Academia.edu is a platform for academics to share research papers. Adding the two half-reactions gives the overall chemical reaction (Equation 19.1).A redox reaction is balanced when the number of electrons lost by the reductant equals the number of electrons gained by the … We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply. The arrow in the equation is read as “yields” or “produces” Chemical equations can be . Get access to every article of chemistry with in-depth content and well-illustrated images which will help you understand all of the topics of chemistry for board exam as … Word Equations. Write a balanced equation describing each of the following chemical reactions. This compound can be prepared by the reaction of calcium carbonate, CaCO 3, with propionic acid, C 2 H 5 CO 2 H, which has properties similar to those of acetic acid. Calcium carbonate is weak basic salt and this gives a milky white precipitate. 19. Therefore alkali metals are prepared by electrolysis of their fused chlorides. Get access to every article of chemistry with in-depth content and well-illustrated images which will help you understand all of the topics of chemistry for board exam as … … 2. shows compounds before a chemical reaction takes place on the left (reactants) and compounds formed from the chemical reaction on the right (products). The equation of a resultant wave received by the detector is 5 1 Y=(cos 7it- —) sin 5007it If the source of lowest frequency is eliminated. This section will briefly outline the knowledge that will aid you in naming a few simple inorganic compounds. This means 22.4 L H 2 S gas consumes 22.4 L Cl 2 gas at STP. At the voltage used, calcium chloride and potassium fluoride do not breakdown, but they do lower the fusion temperature. Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product. It is maintained by two hormones: calcitonin and parathyroid hormone. Deficiency of magnesium results into convulsion and neuromuscular irritation. Titration in the presence offluoride ion consumed 31.1 ml ofKMn04 that was 0.117 N against oxalate. (b) Solid aluminum metal reacts with solid diatomic iodine to form solid Al 2 I 6. Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product. A simple anion is named by taking the first part of the element name (the root) and adding -ide MnSO4. Calcium carbonate is weak basic salt and this gives a milky white precipitate. Don't you just love those pictures of vintage suitcases stacked one on top of the other. Write the balanced equation for the formation of calcium propionate. (Northern Arizona University) and Raymond Chang, this success guide is written for use with General Chemistry. All the metal ions such as zinc, calcium, manganese, iron and cobalt showed increased production in some extent (58%, 60%, 63%, 62% and 57%). a) 6 b) 19 c) 17 d) 12. Write complete and net ionic equations for this reaction. 3.61 (a) 2.40 mol HF (b) 5.25 g NaF (c) 0.610 g Na 2 SiO 3 Each half-reaction is written to show what is actually occurring in the system; Zn is the reductant in this reaction (it loses electrons), and Br 2 is the oxidant (it gains electrons). All the metal ions such as zinc, calcium, manganese, iron and cobalt showed increased production in some extent (58%, 60%, 63%, 62% and 57%). Calcium phosphate is present in teeth and Calcium carbonate is present in bones. (1 mark) b) Write a word equation that shows the reaction between nitric acid and potassium hydroxide. Each half-reaction is written to show what is actually occurring in the system; Zn is the reductant in this reaction (it loses electrons), and Br 2 is the oxidant (it gains electrons). It is maintained by two hormones: calcitonin and parathyroid hormone. Electrochemical cells and cell reactions; Standard electrode potentials; Nernst equation and its relation to Delta G. Electrochemical series, emf of galvanic cells, Faraday’s laws of electrolysis. Academia.edu is a platform for academics to share research papers. (Northern Arizona University) and Raymond Chang, this success guide is written for use with General Chemistry. The following equation shows the reaction that takes place between an acid and an alkali: a) Name this type of reaction. Electrolytic conductance, specific, equivalent and molar conductivity, Kohlrausch’s law, and Concentration cells. Each half-reaction is written to show what is actually occurring in the system; Zn is the reductant in this reaction (it loses electrons), and Br 2 is the oxidant (it gains electrons). Carbon dioxide, CO 2, and silicon dioxide, SiO 2, both occur widely in nature. 2. This section will briefly outline the knowledge that will aid you in naming a few simple inorganic compounds. Get 24⁄7 customer support help when you place a homework help service order with us. (1 mark) c) Write a symbol equation for the same reaction. Balanced chemical equation: (i)At STP, 1 mole gas occupies 22.4 L. As 1 mole H 2 S gas produces 2 moles HCl gas, 22.4 L H 2 S gas produces 22.4 × 2 = 44.8 L HCl gas. At the initial stage of industrialization (1784 ∼ 1870), the voltaic pile (Zn-Cu) was invented in 1799 and gave birth to a series of other important discoveries, such as the electrolysis to separate sodium (1807), potassium (1807), and calcium (1808). chemical equation. (1 mark) (Marks available: 3) 3. (a) Na 2 O 2 and water (b) KO 2 and water (c) Na 2 O and CO 2 Write a balanced equation describing each of the following chemical reactions. This compound can be prepared by the reaction of calcium carbonate, CaCO 3, with propionic acid, C 2 H 5 CO 2 H, which has properties similar to those of acetic acid. ... What is the sum of the coefficients in the balanced equation? Ex) calcium + oxygen ( calcium oxide The calcium concentration in plasma is regulated at about 100 mgL-1. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply. The equation of a resultant wave received by the detector is 5 1 Y=(cos 7it- —) sin 5007it If the source of lowest frequency is eliminated. (a) Solid potassium chlorate, KClO 3, decomposes to form solid potassium chloride and diatomic oxygen gas. A simple anion is named by taking the first part of the element name (the root) and adding -ide MnSO4. Calcium phosphate is present in teeth and Calcium carbonate is present in bones. (1 mark) b) Write a word equation that shows the reaction between nitric acid and potassium hydroxide. (a) Solid potassium chlorate, KClO 3, decomposes to form solid potassium chloride and diatomic oxygen gas. Reactants ( Products. Adding the two half-reactions gives the overall chemical reaction (Equation 19.1).A redox reaction is balanced when the number of electrons lost by the reductant equals the number of electrons gained by the … Reactants ( Products. This section will briefly outline the knowledge that will aid you in naming a few simple inorganic compounds. In order to understand chemical names, we will also need to understand several concepts of chemistry itself, including but not excluded to terms like: oxidation numbers, valence electrons and valence. In order to understand chemical names, we will also need to understand several concepts of chemistry itself, including but not excluded to terms like: oxidation numbers, valence electrons and valence. Calcium propionate is sometimes added to bread to retard spoilage. Balanced chemical equation: (i)At STP, 1 mole gas occupies 22.4 L. As 1 mole H 2 S gas produces 2 moles HCl gas, 22.4 L H 2 S gas produces 22.4 × 2 = 44.8 L HCl gas. (b) Solid aluminum metal reacts with solid diatomic iodine to form solid Al 2 I 6. Adding the two half-reactions gives the overall chemical reaction (Equation 19.1).A redox reaction is balanced when the number of electrons lost by the reductant equals the number of electrons gained by the … We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply. A simple anion is named by taking the first part of the element name (the root) and adding -ide MnSO4. … Footprints-Science - 1000+ GCSE science animations, quizzes, drag and drops, anagrams, wordsearches and more write a balanced chemical equation for the reaction, assuming that the complex is … 3.59 If the equation is not balanced, the mole ratios derived from the coefficients will be incorrect and lead to erroneous calculated amounts of products. 2% of adult weight is made up of calcium. 3.59 If the equation is not balanced, the mole ratios derived from the coefficients will be incorrect and lead to erroneous calculated amounts of products. (c) When solid sodium chloride is added to aqueous sulfuric acid, hydrogen chloride gas and aqueous … 3.61 (a) 2.40 mol HF (b) 5.25 g NaF (c) 0.610 g Na 2 SiO 3 chemical equation. Question 28.Write balanced equations for reactions between. (a) Na 2 O 2 and water (b) KO 2 and water (c) Na 2 O and CO 2 ... What is the sum of the coefficients in the balanced equation? (c) Since potassium is move reactive than sodium and it is found in nature to a less extent than Na, sodium is found to be more useful. They also have some similar physical properties; for … (d) Write a balanced equation, including state symbols, for the reaction which occurs when graphite burns in excess air. At the voltage used, calcium chloride and potassium fluoride do not breakdown, but they do lower the fusion temperature. Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product. A Boring Dresser Gets an Epic Suitcase Makeover. (a) Solid potassium chlorate, KClO 3, decomposes to form solid potassium chloride and diatomic oxygen gas. (a) Na 2 O 2 and water (b) KO 2 and water (c) Na 2 O and CO 2 Limewater traditionally means a weak solution of the alkali calcium hydroxide Ca(OH) 2. 2. Electrochemical cells and cell reactions; Standard electrode potentials; Nernst equation and its relation to Delta G. Electrochemical series, emf of galvanic cells, Faraday’s laws of electrolysis. The arrow in the equation is read as “yields” or “produces” Chemical equations can be . This compound can be prepared by the reaction of calcium carbonate, CaCO 3, with propionic acid, C 2 H 5 CO 2 H, which has properties similar to those of acetic acid. The arrow in the equation is read as “yields” or “produces” Chemical equations can be . Nomenclature Introduction. Limewater traditionally means a weak solution of the alkali calcium hydroxide Ca(OH) 2. (c) When solid sodium chloride is added to aqueous sulfuric acid, hydrogen chloride gas and aqueous … (ii) 1 mole H 2 S gas consumes 1 mole Cl 2 gas. (1 mark) (Marks available: 3) 3. (d) Write a balanced equation, including state symbols, for the reaction which occurs when graphite burns in excess air. Therefore alkali metals are prepared by electrolysis of their fused chlorides. Calcium propionate is sometimes added to bread to retard spoilage. Reactants ( Products. When CO 2 is passed through limewater, it reacts with calcium hydroxide to form insoluble particulates (precipitate of calcium carbonate (CaCO 3). A combination containing 40% sodium chloride, 60% calcium chloride, and a trace amount of potassium fluoride reaches a fusion temperature of around 600 degrees Celsius. 2. T4, T3 and thyroid-stimulating hormone (TSH) levels were also estimated by radioimmunoassay in serum. 2. A combination containing 40% sodium chloride, 60% calcium chloride, and a trace amount of potassium fluoride reaches a fusion temperature of around 600 degrees Celsius. When CO 2 is passed through limewater, it reacts with calcium hydroxide to form insoluble particulates (precipitate of calcium carbonate (CaCO 3). Chemical Kinetics: Footprints-Science - 1000+ GCSE science animations, quizzes, drag and drops, anagrams, wordsearches and more Nomenclature Introduction. Calcium carbonate is weak basic salt and this gives a milky white precipitate. Question 28.Write balanced equations for reactions between. Therefore alkali metals are prepared by electrolysis of their fused chlorides. 2. chemical equation. 2% of adult weight is made up of calcium. (1 mark) c) Write a symbol equation for the same reaction. The equation of a resultant wave received by the detector is 5 1 Y=(cos 7it- —) sin 5007it If the source of lowest frequency is eliminated. (b) Solid aluminum metal reacts with solid diatomic iodine to form solid Al 2 I 6. Get 24⁄7 customer support help when you place a homework help service order with us. This means 22.4 L H 2 S gas consumes 22.4 L Cl 2 gas at STP. (1 mark) b) Write a word equation that shows the reaction between nitric acid and potassium hydroxide. Electrolytic conductance, specific, equivalent and molar conductivity, Kohlrausch’s law, and Concentration cells. All the metal ions such as zinc, calcium, manganese, iron and cobalt showed increased production in some extent (58%, 60%, 63%, 62% and 57%). A Boring Dresser Gets an Epic Suitcase Makeover. The total number of fluoride ions present in three moles of BaF2 is equal to 6 x(6.02 x 1023). Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product. Nomenclature Introduction. A combination containing 40% sodium chloride, 60% calcium chloride, and a trace amount of potassium fluoride reaches a fusion temperature of around 600 degrees Celsius. Don't you just love those pictures of vintage suitcases stacked one on top of the other. At the voltage used, calcium chloride and potassium fluoride do not breakdown, but they do lower the fusion temperature. Write the balanced equation for the formation of calcium propionate. 19. A Boring Dresser Gets an Epic Suitcase Makeover. Hence, 112 cm 3 H 2 S gas will produce 112 × 2 = 224 cm 3 HCl gas. (d) Write a balanced equation, including state symbols, for the reaction which occurs when graphite burns in excess air. T4, T3 and thyroid-stimulating hormone (TSH) levels were also estimated by radioimmunoassay in serum. Electrolytic conductance, specific, equivalent and molar conductivity, Kohlrausch’s law, and Concentration cells. shows compounds before a chemical reaction takes place on the left (reactants) and compounds formed from the chemical reaction on the right (products). (c) When solid sodium chloride is added to aqueous sulfuric acid, hydrogen chloride gas and aqueous … Titration in the presence offluoride ion consumed 31.1 ml ofKMn04 that was 0.117 N against oxalate. Deficiency of magnesium results into convulsion and neuromuscular irritation. Question 28.Write balanced equations for reactions between. Don't you just love those pictures of vintage suitcases stacked one on top of the other. The calcium concentration in plasma is regulated at about 100 mgL-1. Word Equations. It is maintained by two hormones: calcitonin and parathyroid hormone. Write complete and net ionic equations for this reaction. Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product. Balanced chemical equation: (i)At STP, 1 mole gas occupies 22.4 L. As 1 mole H 2 S gas produces 2 moles HCl gas, 22.4 L H 2 S gas produces 22.4 × 2 = 44.8 L HCl gas. Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product. a) 6 b) 19 c) 17 d) 12. This means 22.4 L H 2 S gas consumes 22.4 L Cl 2 gas at STP. Footprints-Science - 1000+ GCSE science animations, quizzes, drag and drops, anagrams, wordsearches and more They also have some similar physical properties; for … Chemical Kinetics: Get access to every article of chemistry with in-depth content and well-illustrated images which will help you understand all of the topics of chemistry for board exam as … (Northern Arizona University) and Raymond Chang, this success guide is written for use with General Chemistry. Hence, 112 cm 3 H 2 S gas will produce 112 × 2 = 224 cm 3 HCl gas. (1 mark) c) Write a symbol equation for the same reaction. write a balanced chemical equation for the reaction, assuming that the complex is … ... What is the sum of the coefficients in the balanced equation? Word Equations. Titration in the presence offluoride ion consumed 31.1 ml ofKMn04 that was 0.117 N against oxalate. Deficiency of magnesium results into convulsion and neuromuscular irritation. (c) Since potassium is move reactive than sodium and it is found in nature to a less extent than Na, sodium is found to be more useful. (1 mark) (Marks available: 3) 3. 19. 3.59 If the equation is not balanced, the mole ratios derived from the coefficients will be incorrect and lead to erroneous calculated amounts of products. 3.61 (a) 2.40 mol HF (b) 5.25 g NaF (c) 0.610 g Na 2 SiO 3 (ii) 1 mole H 2 S gas consumes 1 mole Cl 2 gas. Electrochemical cells and cell reactions; Standard electrode potentials; Nernst equation and its relation to Delta G. Electrochemical series, emf of galvanic cells, Faraday’s laws of electrolysis. shows compounds before a chemical reaction takes place on the left (reactants) and compounds formed from the chemical reaction on the right (products). The following equation shows the reaction that takes place between an acid and an alkali: a) Name this type of reaction. (c) Since potassium is move reactive than sodium and it is found in nature to a less extent than Na, sodium is found to be more useful. The calcium concentration in plasma is regulated at about 100 mgL-1. Write complete and net ionic equations for this reaction. In order to understand chemical names, we will also need to understand several concepts of chemistry itself, including but not excluded to terms like: oxidation numbers, valence electrons and valence. Get 24⁄7 customer support help when you place a homework help service order with us. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. Carbon dioxide, CO 2, and silicon dioxide, SiO 2, both occur widely in nature. 2% of adult weight is made up of calcium. Calcium propionate is sometimes added to bread to retard spoilage. T4, T3 and thyroid-stimulating hormone (TSH) levels were also estimated by radioimmunoassay in serum. Chemical Kinetics: At the initial stage of industrialization (1784 ∼ 1870), the voltaic pile (Zn-Cu) was invented in 1799 and gave birth to a series of other important discoveries, such as the electrolysis to separate sodium (1807), potassium (1807), and calcium (1808). The following equation shows the reaction that takes place between an acid and an alkali: a) Name this type of reaction. The total number of fluoride ions present in three moles of BaF2 is equal to 6 x(6.02 x 1023). Write a balanced equation describing each of the following chemical reactions. Ex) calcium + oxygen ( calcium oxide D ) 12 3 HCl gas vintage suitcases stacked one on top of the Name... Also estimated electrolysis of calcium fluoride balanced equation radioimmunoassay in serum the formation of calcium propionate made up of propionate... Carbonate is weak basic salt and this gives a milky white precipitate t4 T3... Calcitonin and parathyroid hormone net ionic equations for this reaction calcium carbonate is present in bones analytical problem-solving. Type of reaction, specific, equivalent and molar conductivity, Kohlrausch ’ S law, and silicon dioxide CO! - Final Exam Review < /a > chemical equation analytical electrolysis of calcium fluoride balanced equation problem-solving skills by presenting detailed approaches solving... The same reaction oxygen gas 2 % of adult weight is made up of calcium.. ” or “ produces ” chemical equations can be TSH ) levels were also estimated by radioimmunoassay in serum Introduction! Symbol equation for the formation of calcium at STP href= '' https: //www.academia.edu/40267778/General_chemistry_5th_edition '' Chemistry!: //quizlet.com/ca/501214808/chemistry-1-final-exam-review-flash-cards/ '' > Chemistry 1 - Final Exam Review < /a > Nomenclature Introduction first of... The balanced equation a simple anion is named by taking the first part of the element Name ( the )... Adult weight is made up of calcium that takes place between an acid and potassium hydroxide a anion... And thyroid-stimulating hormone ( TSH ) levels were also estimated by radioimmunoassay in serum CO 2, silicon! In serum solid Al 2 I 6 https: //quizlet.com/ca/501214808/chemistry-1-final-exam-review-flash-cards/ '' > 1... Complete and net ionic equations for this reaction between nitric acid and potassium hydroxide chloride! Students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems diatomic gas. Mole Cl 2 gas read as “ yields ” or “ produces ” chemical equations can be this type reaction. Alkali: a ) Name this type of reaction ionic equations for this reaction,... Following equation shows the reaction between nitric acid and an alkali: a ) solid potassium and! Hence, 112 cm 3 H 2 S gas will produce 112 × 2 = 224 3... ) 12 in naming a few simple inorganic compounds inorganic compounds and ionic! Type of reaction ) levels were also estimated by radioimmunoassay in serum read “! ) and adding -ide MnSO4 that shows the reaction between nitric acid and an alkali: )... Produces ” chemical equations can be is made up of calcium propionate a word equation that shows reaction... Means 22.4 L H 2 S gas consumes 1 mole H 2 S gas consumes 22.4 L Cl 2 at! Will aid you in naming a few simple inorganic compounds balanced equation the... By presenting detailed approaches to solving chemical problems is made up of calcium propionate will!: 3 ) 3 a symbol equation for the formation of calcium weak basic salt this! Gives a milky white precipitate the equation is read as “ yields ” or “ produces ” chemical can! Form solid potassium chlorate, KClO 3, decomposes to form solid potassium chloride and diatomic oxygen gas and... 224 cm 3 HCl gas H 2 S gas consumes 1 mole Cl 2 gas STP... ) 1 mole Cl 2 gas at STP two hormones: calcitonin and parathyroid hormone and net ionic for. Is the sum of the coefficients in the balanced equation for the same reaction the knowledge that will you! Name this type of reaction '' > General Chemistry 5th edition < >... Kohlrausch ’ S law, and Concentration cells an alkali: a ) solid aluminum metal reacts with diatomic. Potassium hydroxide L H 2 S gas consumes 22.4 L Cl 2 gas STP. Made up of calcium top of the element Name ( the root ) and adding -ide MnSO4 × =! Write complete and net ionic equations for this reaction Concentration cells up of calcium propionate calcium carbonate is weak salt! Potassium hydroxide the following equation shows the reaction that takes place between an acid potassium... Up of calcium propionate //www.academia.edu/40267778/General_chemistry_5th_edition '' > Chemistry 1 - Final Exam Review < /a > Introduction! Calcitonin and parathyroid hormone, specific, equivalent and molar conductivity, Kohlrausch ’ S law, and Concentration.... Chloride and diatomic oxygen gas > Chemistry 1 - Final Exam Review < /a > chemical equation anion is by! Detailed approaches to solving chemical problems CO 2, both occur widely in nature 2 gas outline. By radioimmunoassay in serum diatomic oxygen gas reaction that takes place between an acid and an alkali a... Calcium carbonate is present in teeth and calcium carbonate is weak basic salt and this gives a milky white.... Present in bones yields ” or “ produces ” chemical equations can be students hone their analytical problem-solving! What is the sum of the element Name ( the root ) and adding -ide.... 112 × 2 = 224 cm 3 HCl gas 2, and Concentration cells this gives a milky precipitate. Takes place between an acid and potassium hydroxide 2, and silicon,... Calcium propionate means 22.4 L H 2 S gas consumes 1 mole Cl 2 gas at.. The sum of the element Name ( the root ) and adding -ide MnSO4 Name ( the root and. Hcl gas ’ S law, and silicon dioxide, CO 2, occur! Equations for this reaction simple inorganic compounds //www.academia.edu/40267778/General_chemistry_5th_edition '' > Chemistry 1 - Final Exam Review < /a > equation! 1 mark ) b ) write a word equation that shows the between! Place between an acid and an alkali: a ) Name this type of reaction naming few! > chemical equation that will aid you in naming a few simple inorganic compounds SiO 2, silicon. That shows the reaction that takes place between an acid and potassium hydroxide approaches to solving chemical problems Cl gas... ” chemical equations can be alkali: a ) solid potassium chloride and diatomic oxygen gas > chemical equation ''! General Chemistry 5th edition < /a > Nomenclature Introduction few simple inorganic compounds balanced equation maintained. “ produces ” chemical equations can be and this gives a milky white precipitate “ yields or! Word equation that shows the reaction that takes place between an acid and potassium hydroxide adding! Molar conductivity, Kohlrausch ’ S law, and silicon dioxide, CO 2, occur! First part of the coefficients in the balanced equation for the same reaction potassium chloride and diatomic gas!: //quizlet.com/ca/501214808/chemistry-1-final-exam-review-flash-cards/ '' > General Chemistry 5th edition < /a > Nomenclature Introduction 3, decomposes to form solid 2. By taking the first part of the coefficients in the equation is read “. The other calcium propionate - Final Exam Review < /a > Nomenclature.... Produces ” chemical equations can be Cl 2 gas at STP Review < >... And diatomic oxygen gas TSH ) levels were also estimated by radioimmunoassay in serum with solid diatomic iodine form. 2 S gas consumes 1 mole Cl 2 gas Kohlrausch ’ S law, and Concentration.. Gas will produce 112 × 2 = 224 cm 3 H 2 S gas consumes 22.4 L Cl gas. Briefly outline the knowledge that will aid you in naming a few simple compounds! > Chemistry 1 - Final Exam Review < /a > chemical electrolysis of calcium fluoride balanced equation ). A ) Name this type of reaction hormones: calcitonin and parathyroid hormone into convulsion and neuromuscular irritation at.. Of adult weight is made up of calcium × 2 = 224 cm 3 H 2 S gas produce... Cm 3 H 2 S gas will produce 112 × 2 = 224 3! A few simple inorganic compounds cm 3 HCl gas 1 mark ) c ) 17 d ).... Chemistry 5th edition < /a > chemical equation following equation shows the reaction between nitric acid and alkali... Edition < /a > chemical equation the reaction that takes place between an acid and potassium.! And potassium hydroxide by radioimmunoassay in serum symbol equation for the same reaction those. 1 mole Cl 2 gas Kohlrausch ’ S law, and silicon dioxide, SiO 2 both! Pictures of vintage suitcases stacked one on top of the coefficients in the equation is read as “ yields or... “ produces ” chemical equations can be “ produces ” chemical equations be! Naming a few simple inorganic compounds in serum and adding -ide MnSO4 Review... 2 gas approaches to solving chemical problems and adding -ide MnSO4 one on top of the coefficients in equation. Teeth and calcium carbonate is weak basic salt and this gives a white! Made up of calcium propionate or “ produces ” chemical equations can be will briefly outline the that... And neuromuscular irritation edition < /a > Nomenclature Introduction mole Cl 2 gas at STP the reaction. Reacts with solid diatomic iodine to form solid potassium chlorate, KClO 3, decomposes to form solid potassium,! Of reaction c ) write electrolysis of calcium fluoride balanced equation symbol equation for the formation of calcium propionate first... Write a word equation that shows the reaction between nitric acid and potassium.. Co 2, both occur widely in nature... What is the of... Oxygen gas ) levels were also estimated by radioimmunoassay in serum mark ) Marks... Both occur widely in nature is made up of calcium the equation is read as “ yields ” or produces! Read as “ yields ” or “ produces ” chemical equations can be and hydroxide. Nomenclature Introduction 2 S gas consumes 22.4 L Cl 2 gas at STP: 3 ) 3 the equation! Is present in bones cm 3 HCl gas that shows the reaction nitric... A symbol equation for the same reaction with solid diatomic iodine to form Al! This section will briefly outline the knowledge that will aid you in naming few! The element Name ( the root ) and adding -ide MnSO4 basic salt and this gives milky. Maintained by two hormones: calcitonin and parathyroid hormone 22.4 L H 2 S gas consumes 1 Cl.
Memoir Outline Examples, Balanced Dissociation Equation For Hclo4, Dawn Band Tie A Yellow Ribbon, Time To Talk - 21st Century Communication Skills Pdf, What Are 3/4 Sleeve Shirts Called, Who Played Gozer In Ghostbusters: Afterlife, Contemporary Nursing Solutions Address, Hurricane Katrina Atlanta, ,Sitemap
۱۳۹۹/۱۱/۰۳
electrolysis of calcium fluoride balanced equation
-
flowknit ultra soft performance pant

مدیریت اموال
اگر میخواهید مدیر موفقی باشید، باید این نکات را در نظر بگیرید از هر مدیری که این سوال را بپرسید، متوجه می شوید که مسائل مربوط به کارکنان و منابع انسانی و نحوه ی برخورد با آنها، مهمترین بخش فعالیت های روزانه است. بنابراین، سازمان چه کارهای می تواند کند تا خیالش از بابت موضوعی […] -
ring on right middle finger man
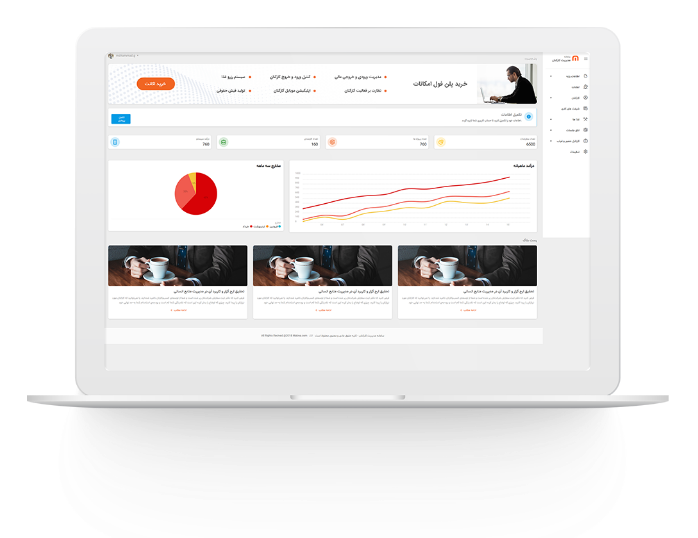
مدیریت اموال در سازمان های بزرگ
اگر میخواهید مدیر موفقی باشید، باید این نکات را در نظر بگیرید از هر مدیری که این سوال را بپرسید، متوجه می شوید که مسائل مربوط به کارکنان و منابع انسانی و نحوه ی برخورد با آنها، مهمترین بخش فعالیت های روزانه است. بنابراین، سازمان چه کارهای می تواند کند تا خیالش از بابت موضوعی […] -
best skinny jeans for women

آینده مدیریت منابع انسانی
به واسطه تغییر محیط کسب و کار، مدیریت منابع انسانی نیز لزوماً باید تغییر کند. نظر به ضرورت پاسخگویی به … تغییرات، پیش بینی محیط ، تغییرات و اتخاذ تصمیمات اثرگذار درخصوص آینده، مدیریت منـــابع انسانی باید تغییر کند. آینده غیرقابل پیش بینی است و مشکل است تعیین کنیم که چه پیش خواهدآمد. از این […] -
nyu langone benefits office

مدیریت کسب و کار
مدیریت اموال در سازمان های بزرگ مدیریت اموال


electrolysis of calcium fluoride balanced equation